Biologically Ocurring Deals-Alder Reactions- Lovastatin -
Lovastatin is a fungal metabolite isolated from cultures of Aspergillus terreus. The compound is a potent anticholesteremic agent, inhibitor of 3-hydroxy-3methylglutaryl-coenzyme A reductase (HMG-CoA reductase), an enzyme which catalyzes the conversion of HMG-CoA to mevalonate. Mevalonate is a required building block for cholesterol biosynthesis and lovastatin interferes with its production by acting as a competitive inhibitor for HMG-CoA which binds to the HMG-CoA reductase which is the rate-limiting enzyme in cholesterol biosynthesis. It also stimulates the production of low-density lipoprotein receptors in the liver.
Lovastatin, is inactive in the native form, the form in which it is administered, is hydrolyzed to the β-hydroxy acid form in the body and it is this form which is active. Presumably, the reductase acts on the hydrolyzed lovastatin to reduce the carboxylic acid moiety.
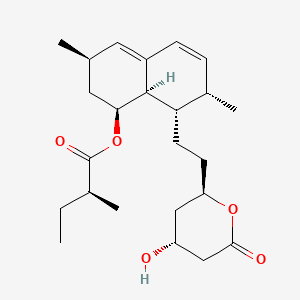
IUPAC
Name:
[(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl]
(2S)-2-methylbutanoate
Molecular
Formula: C24H36O5 Molecular Weight:
404.53964
Other Common names are : Mevinolin, Mevacor, Monacolin K, Altoprev, Lovalord, Mevinacor, Nergadan, Altocor, Artein
In vitro formation of a
triketide lactone using a genetically-modified protein derived from
6-deoxyerythronolide B synthase has been demonstrated. The stereochemistry of
the molecule supports the idea that an
enzyme-catalyzed Diels-Alder reaction may occur during assembly of the
polyketide chain. It thus appears that biological Diels-Alder reactions may be
triggered by generation of reactive triene systems on an enzyme surface.


References
http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=53232&loc=ec_rcs
http://chem257.pbworks.com/w/page/15645822/Lovastatin