Ribulose-1,5- bisphosphate carboxylase/ oxygenase
 |
| Active site/ reactions carried out |
 |
| 2D structure |
 |
| Ribulose-1,5- bisphosphate molecule structure |
Ribulose-1,5- bisphosphate carboxylase oxylase
- chemical formula: CH2OHCO(CHOH) 2CH2OH
- molar mass: 310.09 g/mol
- ligands: Mg+2 ion
This enzyme is commonly known by the shorter name RuBisCO, and is an enzyme involved in the Calvin cycle that catalyzes the first major step of carbon fixation, which is the process by which the atoms of atmospheric carbon dioxide are made available to organisms in the form of energy-rich molecules such as glucose. RuBisCO is very important in terms of biological impact because it catalyzes the primary chemical reaction by which inorganic carbon permanently enters the biosphere.
RuBisCO catalyzes either the carboxylation or the oxygenation of ribulose-1,5-bisphosphate (also known as RuBP) with carbon dioxide or oxygen.
.
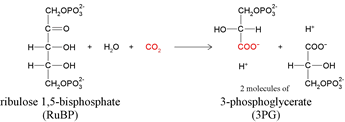 |
| Carboxylation reaction |
 |
| Oxygenation reaction |
The rate of reaction for this enzyme is really slow compared to most enzymes. RuBisCO fixes only about three carbon dioxide molecules per second while most enzymes can process thousands of molecules per second. However plants compensate this slow rate by building lots of it. This enzyme also shows an unfortunate lack of specificity, since molecules of oxygen and carbon dioxide are similar in shape and properties, RuBisCO enzymes occasionally fix oxigen molecules to the sugar chain, forming faulty oxigenated products.
references:
http://en.wikipedia.org/wiki/Ribulose-1,5-bisphosphate
http://guweb2.gonzaga.edu/faculty/cronk/biochem/R-index.cfm?definition=rubisco
http://guweb2.gonzaga.edu/faculty/cronk/biochem/R-index.cfm?definition=rubisco (3D ribbon structure)
http://www.climatewiki.org/wiki/Rubisco
http://www.rcsb.org/pdb/101/motm.do?momID=11
Your molar mass is that of the organic substrate, not the enzyme itself, which should be on the order of tens of thousands!
ReplyDeleteJesus,
ReplyDeleteHere is my question:
If the heat from bruning 7.800g of C6H6 is added to 2.387 kg of water that is initially at a temperature of 21 degrees C, what is the final temperature of the water? The specific heat of liquid is 4.184 J/g*Degrees C.
Here is my question:
ReplyDeleteIf the heat from bruning 7.800g of C6H6 is added to 2.387 kg of water that is initially at a temperature of 21 degrees C, what is the final temperature of the water? The specific heat of liquid is 4.184 J/g*Degrees C.